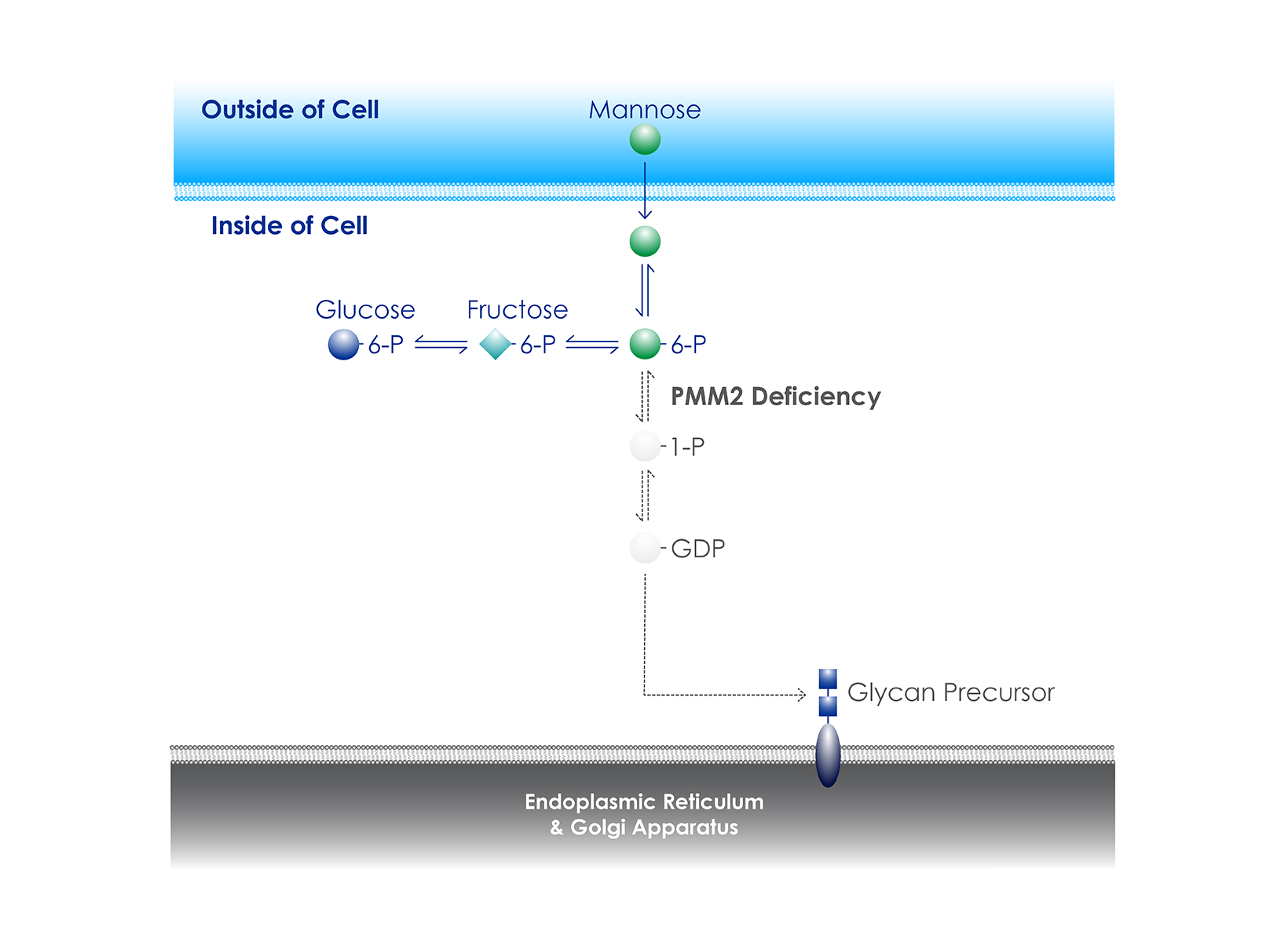
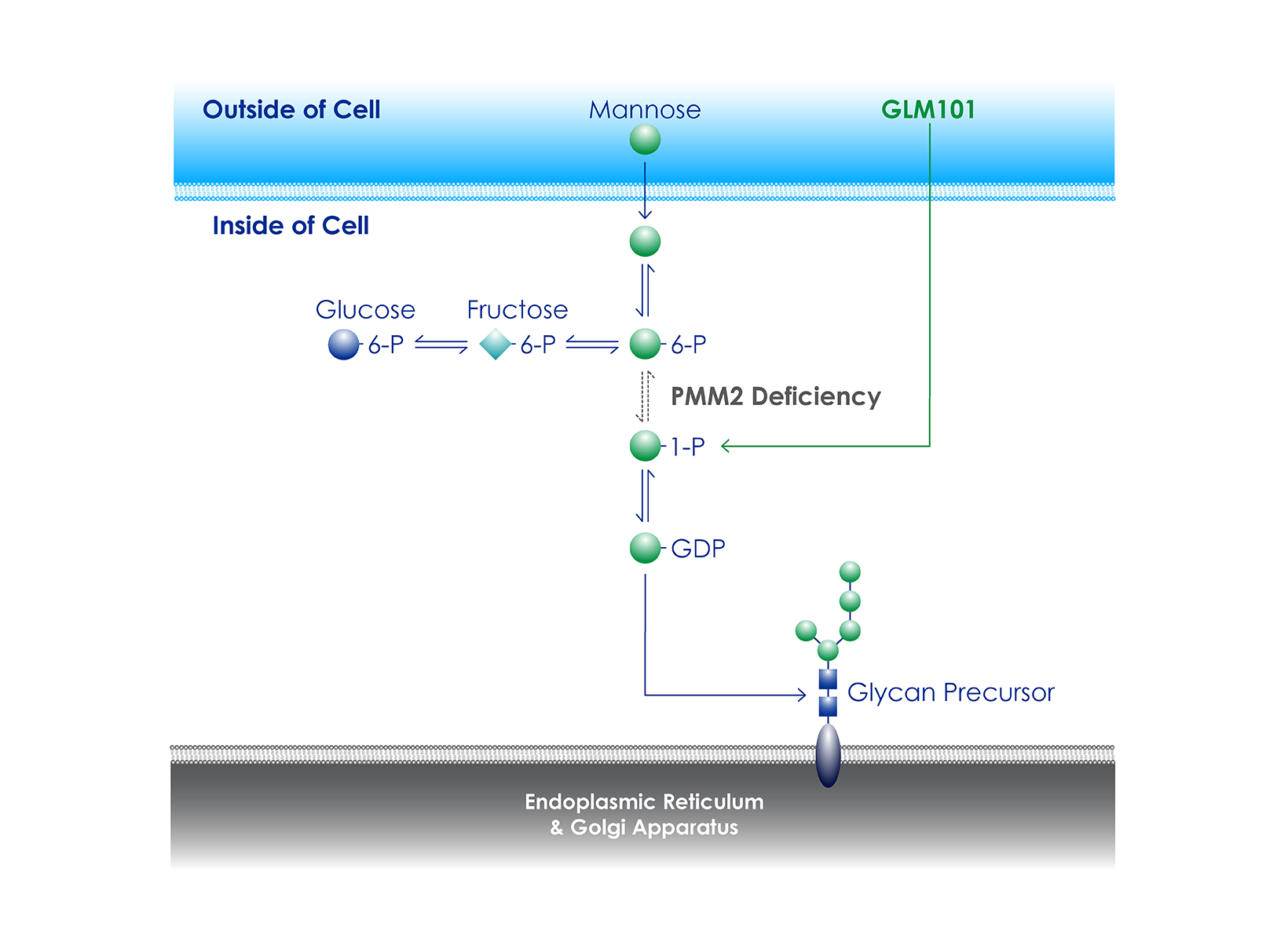
Clinical Trial of GLM101 for The treatment of PMM2-CDG
Glycomine has received U.S. Food and Drug Administration (FDA) clearance of an Investigational New Drug (IND) application for GLM101 for the treatment of PMM2-CDG. The company is now enrolling study participants in POLAR, a global, randomized, placebo-controlled Phase 2b clinical trial to assess the safety and efficacy of GLM101 in children and adults with PMM2-CDG (ClinicalTrials.gov Identifier: NCT06892288).